Amadee-20-RETINA
Details
| Acronym | RETINA |
| Description | An eye diagnostic device is tested in Mars analog conditions |
| Principal Investigator (PI) | Dr. med. Claudia Stern | claudia.stern@dlr.de |
| Organisation | German Aerospace Center (DLR), Institute for Aerospace Medicine, Dept. of Clinical Aerospace Medicine |
| Co-Investigators | Scott Ritter German Aerospace Center (DLR), Institute for Aerospace Medicine, Dept. of Clinical Aerospace Medicine Linder Höhe, 51147 Köln, Germany E-Mail: scott.ritter@dlr.de Phone: +49 2203 601 5129 |
Summary
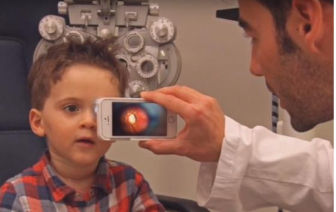
The German Aerospace Center (DLR) has tested eye diagnostic devices for spaceflight applications as tools for detecting and mitigating against the numerous vision pathologies that astronauts experience in the space environment. These vision pathologies have been collectively termed Spaceflight Associated Neuro-ocular Syndrome(SANS). These devices are intended to replace older eye diagnostics devices, which are currently used inspaceflight, by offering multiple advantages in size, weight, and diagnostic capability. DLR’s technology is the result of over 5 years of research and development to provide smaller, lighter, and better performing medical diagnostics technologies for use in space and on Earth. This technology is currently at Technology Readiness Level (TRL)4. The goal of this experiment is to test this device in Marsanalog conditions for potential use on future Mars missions.The objective is to show that small, lightweight, mobile, non-invasive, non-contact, light-based retinal imaging devices can feasibly capture fundus images from healthy test subjects in Mars analog conditions. During the mission, one analog astronaut uses the device to capture retinal images of another crew member, repeated during the beginning, middle, and end of the mission.
Experiment Data
| Date | Files |
|---|---|
| no data received | no files |